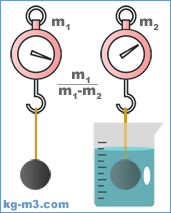
The volumetric mass density (or specific mass) of material is its mass divided with unit of volume. SI unit for density is kg/m3 (the reason for our domain name). The symbol most often used for density is ρ (the lower case of the Greek letter rho), but the Latin letter D can also be used. It is important to understand, that the mass density of a material varies with temperature and pressure, but some materials are less sensitive to these changes. The variance is typically small for solids and liquids but bigger for gasses.
ρ = m / V
where ρ: density, m: mass and V: volume.
On kg-m3.com website you can find density values for over 1000 materials and conversion calculator for every popular mass and volume unit combinations.
What can you do with this data? Check few examples for density calculation. One fo the most popular density unit conversion is pound per cubic inch to kg per liter.
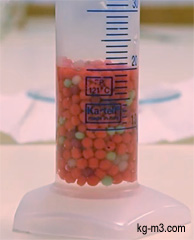
Bulk Density (or volumetric density or apparent density) is a property of powders, granular material and other similar particulate solids (for example soil, gravel). It is defined as the mass of many particles of the material divided by the total volume they occupy.
ρBulk = mg / Vt
where ρBulk: bulk density, mg: mass of granular material, Vt: total volume (particle volume + inter-particle void volume + internal pore volume)

Specific gravity (or relative density) is the ratio of the mass density of a substance to the density (mass of the same unit volume) of a reference substance. In most of the cases the reference substance is water for liquids or air for gases. Specific gravity represents a ratio and is therefore dimensionless. Specific gravity for liquids is nearly always measured with respect to water at 4 °C (39.2 °F). For gases, the reference is air at 20°C (room temperature, 68 °F).
SG = ρsubstance / ρreference
where SG is specific gravity (dimensionless), ρreference - density of reference material (most common ρwater4°C = 999.9749 kg/m3 or ρair20°C= 1.2041 kg/m3)